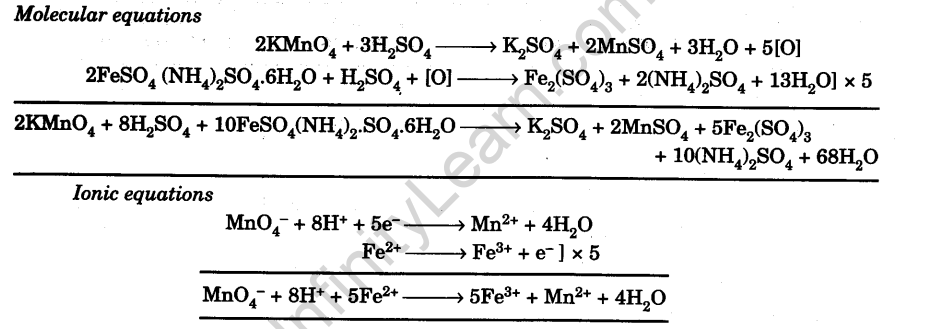
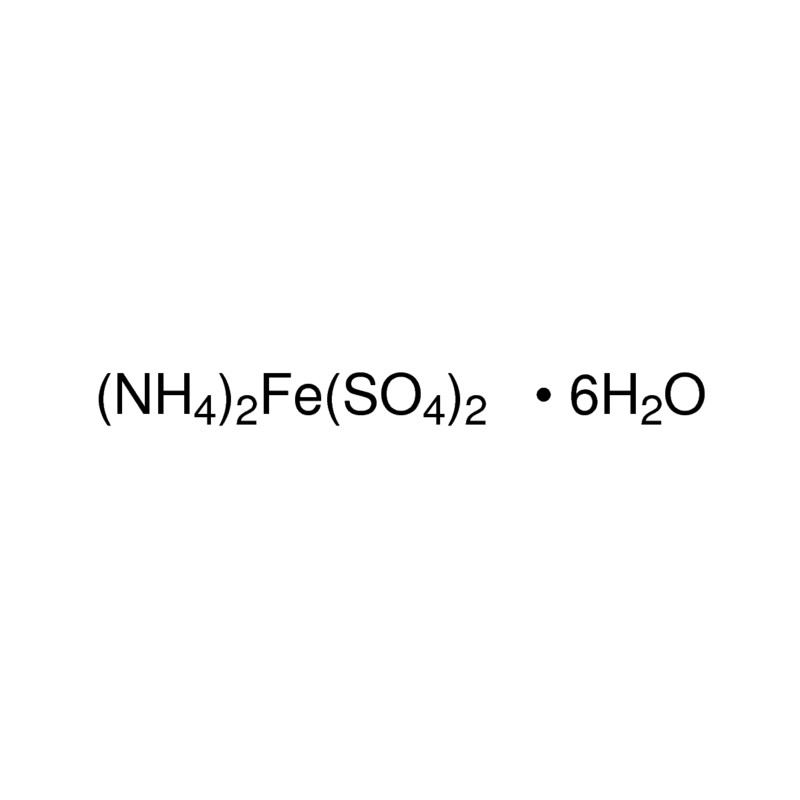The percentage composition of Mohr's salt is 14 32% Fe2+, 9 2% NH4+, 49% SO42- 27 57% H2O - Chemistry - Some Basic Concepts of Chemistry - 10320757 | Meritnation.com

When 3.92 g L ^-1 of sample of Mohr's salt reacts completely with 50 mL N/10 KMnO4 solution. The percentage purity of the sample of Mohr's salt is :

The molecular formula of Mohr's salt is (NH.),SO.FeS0.6H,O(1) Find the number of atoms of each element.(2) - Brainly.in

does mohr's salt contain iron ? write the chemical formula of mohr's salt? from brainly - Brainly.in
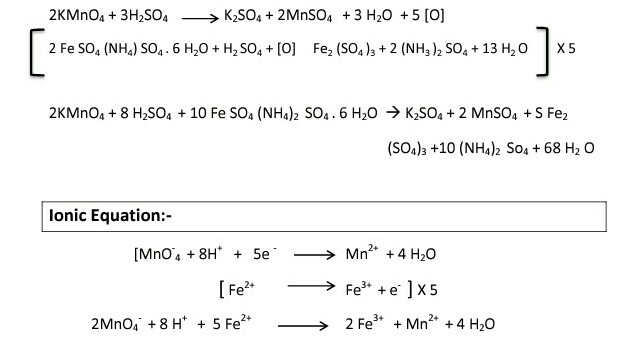







![Solved] Mohr's salt is Solved] Mohr's salt is](https://d10lpgp6xz60nq.cloudfront.net/ss/web/2041461.jpg)