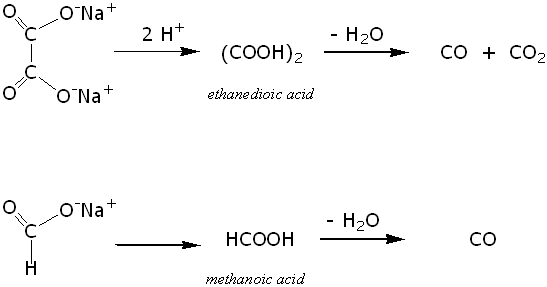
A solution of a salt in concentrated sulphuric acid `H_(2)SO_(4)` acid produced a deep blue colour - YouTube
Why is aniline treated with the excess of concentrated sulfuric acid in the preparation of sulfanilic acid? - Quora

A solution of a salt in concentrated H2SO4 turns a white paste of starch containing potassium iodide blue. The salt may be a:

SOLVED: Identify each as an Acid, Base or Salt then write the equation or reaction that shows how each behaves in water: A) H2SO4 B) NaOH C CaClz D) Mg(OH)2 E) NH3

Assertion: H2SO4 forms only one series of salts.Reasons: The molecules of H2SO4 consists of only one OH group.

SOLVED: What salt is formed in the reaction of silver with sulfuric acid? Write the formula of the salt. Feedback The metal and [ the acid react t0 form a salt and
Sulphuric acid forms two types of salts with an alkali.Give reasons. - Sarthaks eConnect | Largest Online Education Community

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download